Pharmaceutical, Biopharmaceutical / Biotechnology & Nutraceutical
|
SERVICE IS OUR SOLE FOCUS
Competitor filters are taking as long as 6 months to a year to ship – Strainrite can deliver faster. When competitors say “no” to a customized filter, Strainrite welcomes the opportunity. We can supply on time, by manufacturing in the USA in a timely manner. Further our network of Master Distributors are equipped with testing capabilities and keep safety stock in a nearby warehouse. We believe in developing and maintaining long-term, strategic relationships with clients in order to deliver innovative real time solutions to specific customer and market requirements. |
SCIENCE IS OUR SPECIALTY
Strainrite’s experienced personnel possess product knowledge as well as biopharma and regulatory training to assist with a wide range of challenges. Our pharmaceutical grade filters are specialized, validated, designed-for-purpose, QC tested, and clean room manufactured to ISO9001 and cGMP standards. We can help you to optimize a process to filter a batch effectively at the lowest cost per liter. Our consultative approach focuses on custom solutions to filtration problems. We commit the time and resources to tailor our products to our clients’ unique requirements. |
SATISFACTION IS OUR STANDARD
We can help you satisfy regulatory requirements with filters certified to USP standards, validated to perform, factory tested, and testable by the filter user in production. We can satisfy diverse filter needs, from liquid pre- and final-filters to filtration of sterile gases — with a wide array of filter materials to choose from. Extensive research and development, coupled with stringent quality control standards, provides our clients with consistent, reliable filtration products. |
PRIORITY PRODUCTS FOR OPTIMAL FILTRATION

Pur-MAXX SG: 0.2 sterilizing grade 0.2 micron PES membrane filters
• Documented bacterial retention testing • Correlation of challenges to non-destructive integrity tests • LAL testing • Documented cytotoxicity testing • Steam sterilization durability testing • USP Class VI Biological Reactivity In Vivo, testing • USP Physicochemical Testing • Validation guide available on request • Manufacture and testing in third party validated clean rooms in accordance with our ISO 9001-2000 certified Quality Management System • Compliance with the requirements for Class VI 121°C Plastics based on the current USP Biological Reactivity Tests, in vivo. • 100% Integrity tested • USP Bacterial Endotoxins LAL samples tested • Multiple Steam (sterilization cycles — audit testing • USP Oxidizable Substances — audit testing • USP Physicochemical Tests for Plastics — samples tested • Integrity Tested • Water flow — audit tested • Bacterial retention — audit tested • Tested and validated to retain integrity after multiple steam cycles • 316 ss reinforced end treatments • 100% thermally-bonded construction • Absolute-rated dual layer membrane provides reliable, consistent and repeatable filtrate quality • PES membrane offers broad chemical compatibility 
Vent-MAXX Double Layer PTFE: Air & Vent Gas Applications
• Validation Guide for Vent-MAXX Cartridges – available on request • Bacterial challenge testing — liquid “worst case” challenge • Correlation of bacterial challenge to integrity test • Cytoxicity testing • Biological reactivity USP Class VI testing • USP physicochemical tests for plastics testing • Limulus amebocyte lysate (LAL) testing • Integrity-tested at factory • Manufactured and tested in third party validated clean rooms • Fabricated in accordance with our ISO 9001-2000 certified Quality Management System • Tested and complied with the requirements for Class VI 121°C Plastics based on the current USP Biological Reactivity Tests, In Vivo • Tested on sample basis using the Limulus Amebocyte Lysate (LAL) test • Audit tested to verify integrity after exposure to twenty-five (25) one-hour steam cycles at 135°C (275°F) • Samples tested to USP Oxidizable Substances • Samples tested to USP Physicochemical Tests for Plastics • Air flow — pressure-drop audit tested • Bacterial retention audit tested • Tested to demonstrate retention of integrity after multiple steam cycles • 316 ss reinforced end treatments • 100% thermally-bonded construction • Double-layer hydrophobic PTFE membrane for reliability |

Endo-MAXX CN: 0.2 micron charged nylon membrane filters
• Removes charged particles smaller than the absolute retention rating of the filter • Has been applied for reducing endotoxin in water and aqueous fluids • 3rd Party Laboratory Tested • LAL tested • Documented cytotoxicity testing • Validation Guide available upon request • Integrity-tested at factory • Manufactured and tested in third party validated clean rooms • Fabricated in accordance with our ISO 9001-2000 certified Quality Management System • Tested and comply with the requirements for Class VI 121°C Plastics based on the current USP Biological Reactivity Tests, in vivo • Tested on sample basis and determined to contain less than 0.0050 EU/ml using the Limulus Amebocyte Lysate (LAL) test • Audit tested to verify integrity after exposure to twenty (20) one-hour steam cycles at 135°C (275°F) • Samples tested to USP Physicochemical Tests for Plastics • Tested to demonstrate retention of integrity after multiple steam cycles • 316 SS reinforced end treatments • 100% thermally-bonded construction • Nylon 6,6 membrane cast on polyester support for strength • Double-layer membrane for reliability 
MAXX-Cap: single-use / multi-use ultrapure polypropylene capsules
• Great for single use systems • Eliminates CIP (clean in place) expense • Lessens operator exposure to contained compounds • Avoids/reduces capital (stainless steel) investment • Wide range of sizes, filter types, and end fittings are available • Filters are available for installation in the MAXX-CAP capsule that are supported by a full validation guide. • Integrity-tested at factory • Manufactured and tested in third party validated clean rooms • Fabricated in accordance with our ISO 9001-2000 Certified Quality Management System • Tested to comply with the requirements for Class VI 121°C Plastics based on the current USP Biological Reactivity Tests, in vivo • Tested on sample basis using the Limulus Amebocyte Lysate (LAL) test • Audit tested to verify integrity after exposure to twenty-five (25) one-hour steam cycles at 135°C (275°F). • Samples tested to USP Oxidizable Substances • Flow — pressured drop audit tested • Bacterial retention audit tested • Variety of end-fittings available • 100% thermally-bonded construction • Rugged polypropylene outer shell, broadly chemical compatible • Rated to 70 psig • Autoclavable |
|
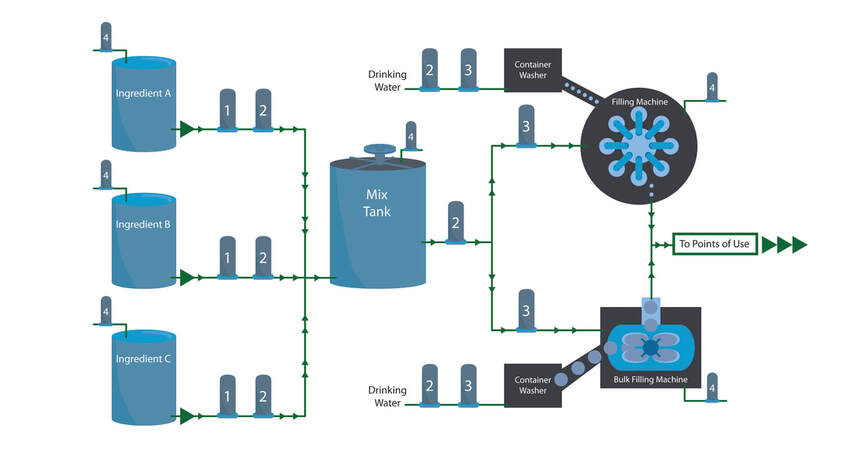
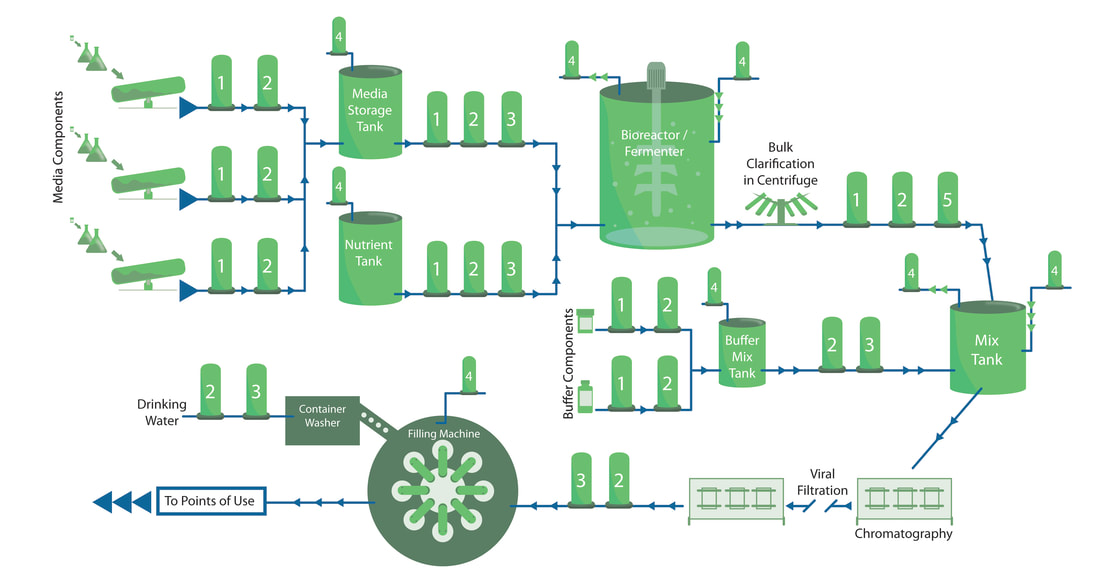
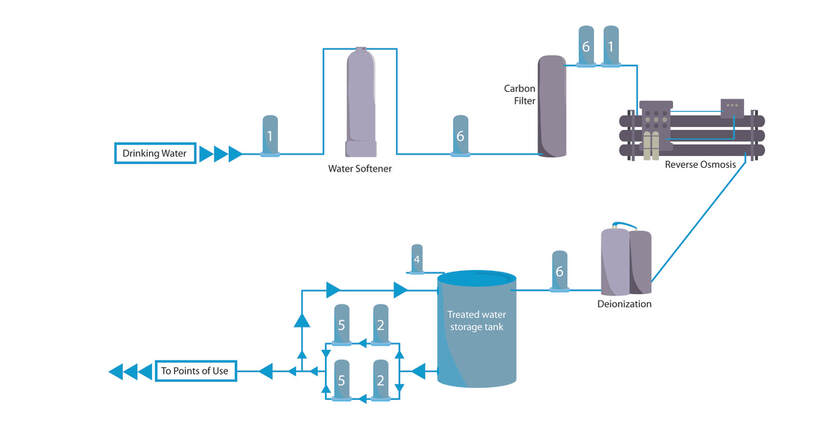
In the pharmaceutical industry, contaminants can have serious consequences. Top notch filtration systems tackle unwanted particles and bacteria so drugs are clean and safe.
For every step in the filtration process (pre-filtration, bioburden reduction, sterilization, tank vent and gas filtration, endotoxin removal and trap filtration), our filters produce the highest quality end results. In short, we use expert science and stringent processes to bring you clean and clear solutions. At Strainrite, we also manufacture a variety of standard design vessels that offer the aesthetics and operational durability of stainless steel at affordable prices. We can also work with you to create custom solutions to meet your needs. Whatever your end goal, we ensure optimal results in all aspects of filtration. When you choose Strainrite, you won’t have to worry about pharmaceutical contamination.You’ll benefit from great outcomes and happy and healthy end users. |
Filtration Solutions For:
|
Filtration of Active Pharmaceutical Ingredients (APIs):Biopharmaceutical Filtration:High Purity Water Filtration: |
1. Prefiltration
Strainrite’s Clarity Depth (2.5” diameter) Clarity Extra Depth (2.7” diameter) and Clarity Continuous Pleated (2.68” diameter) series offer quality depth filtration in an array of filter media:
2. Bioburden Reduction Strainrite’s Clarity Membrane (2.5” diameter) and Clarity Max Membrane (2.7” diameter) series offer quality depth filtration in your choice of filter media:
3. Sterilization Strainrite’s sterile-grade Clarity Mem-Pleat E-SG (2.5” diameter) and Pur-MAXX E-SG (2.7” diameter) filters offer quality depth filtration in a dual-layer pleated polyethersulfone sterile membrane element. 4. Tank Vent & Process Gas Filtration Strainrite offers the highest quality tank vent and process gas filtration with hydrophobic, sterilizing PTFE membrane vent filters, Vent-Rite (single-layer PTFE), and Vent-MAXX (double-layer PTFE) 5. Endotoxin Removal Strainrite offers the highest quality in endotoxin filtration with an absolute-rated, hydrophilic and integrity-tested charged nylon pleated membrane filter, the Endo MAXX CN. 6. Trap Filtration Strainrite offers quality depth trap filtration in an absolute-rated high-solids-loading, polypropylene pleated depth medium with the Pur-Pleat Select (2.5” diameter), the Poly-MAXX Select (2.7” diameter) and the HSLP (Continuous Pleated -2.68” diameter). |
Highest Quality Standard Sanitary Housing:
We manufacture a variety of standard design vessels that offer the aesthetics and operational durability of stainless steel at affordable prices, ensuring optimal cleanability in critical areas.
For special system requirements, our engineers will custom design a system to meet your needs. Strainrite vessels are fabricated from the highest quality materials and conform to strict quality standards.
We manufacture a variety of standard design vessels that offer the aesthetics and operational durability of stainless steel at affordable prices, ensuring optimal cleanability in critical areas.
For special system requirements, our engineers will custom design a system to meet your needs. Strainrite vessels are fabricated from the highest quality materials and conform to strict quality standards.
- Electropolished finish
- Fitted for code 7 & code 6 cartridges
- Stainless steel legs
- Sterilization using hot water or in-line steam